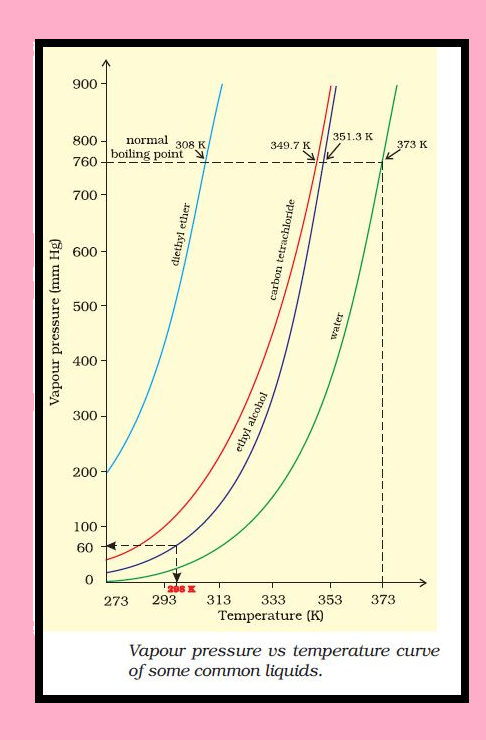
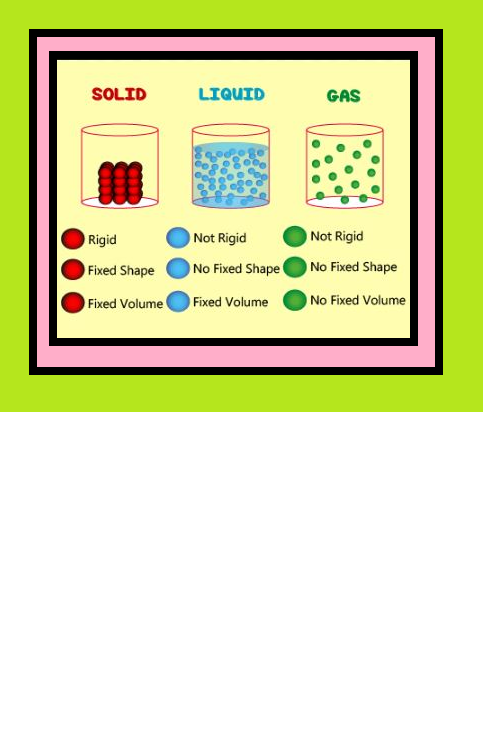
Liquid State :
`=>` Intermolecular forces are stronger in liquid state than in gaseous state.
`=>` Molecules in liquids are so close that there is very little empty space between them and under normal conditions liquids are denser than gases.
`=>` Molecules of liquids are held together by attractive intermolecular forces.
`=>` Liquids have definite volume because molecules do not separate from each other.
`=>` Molecules of liquids can move past one another freely, therefore, liquids can flow, can be poured and can assume the shape of the container in which these are stored.
`=>` Molecules in liquids are so close that there is very little empty space between them and under normal conditions liquids are denser than gases.
`=>` Molecules of liquids are held together by attractive intermolecular forces.
`=>` Liquids have definite volume because molecules do not separate from each other.
`=>` Molecules of liquids can move past one another freely, therefore, liquids can flow, can be poured and can assume the shape of the container in which these are stored.